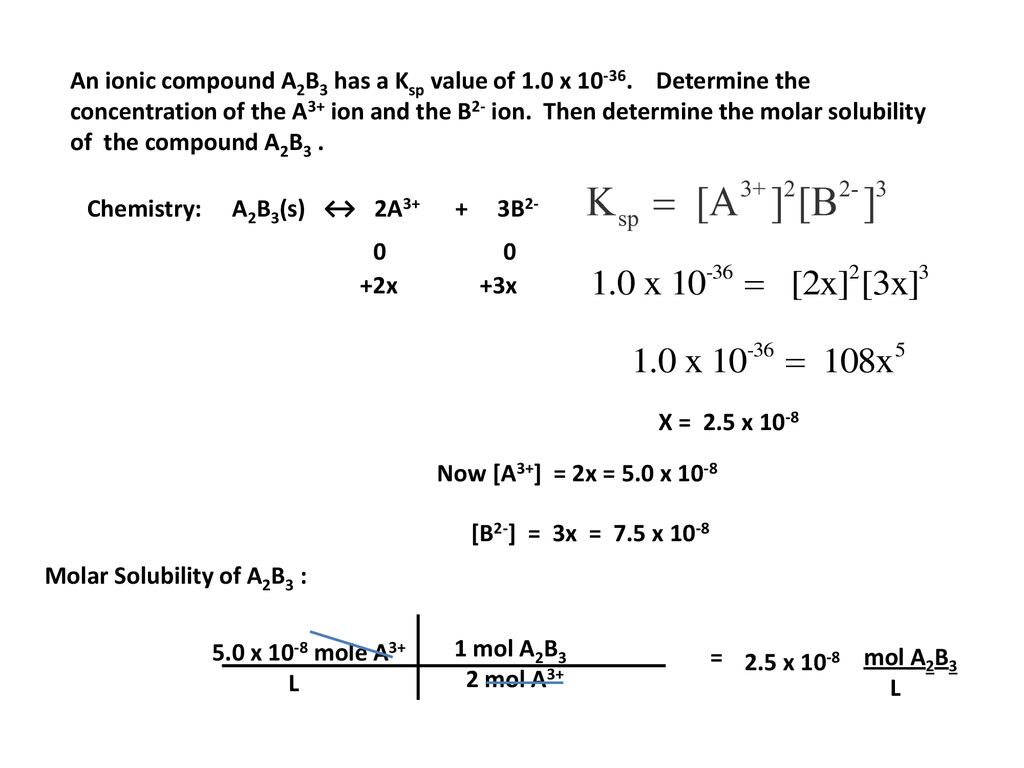
Solubility
Posted: Mon May 08, 2023 2:26 am
Please help me with this task:
The solubility product of As2S3 📖 is 4.0.10−29 (T = 25 °C).
a) Calculate the number of S2- 📖
ions in 70.0 L of saturated As2S3 📖 solution at 25 °C.
b) In 70.0 L of Na2S solution of unknown concentration, a maximum of 0.545 mg of As2S3 📖 can be dissolved at
25 °C. Calculate the mass concentration of the Na2S solution.
Many thanks in advance
The solubility product of As2S3 📖 is 4.0.10−29 (T = 25 °C).
a) Calculate the number of S2- 📖
ions in 70.0 L of saturated As2S3 📖 solution at 25 °C.
b) In 70.0 L of Na2S solution of unknown concentration, a maximum of 0.545 mg of As2S3 📖 can be dissolved at
25 °C. Calculate the mass concentration of the Na2S solution.
Many thanks in advance