Reaction
Moderators: Xen, expert, ChenBeier
-
popabiancamaria
- Sr. Member

- Posts: 29
- Joined: Thu Nov 24, 2011 2:21 pm
- Location: ROMANIA,Bucharest
Acetylene will not react with acetone under ordinary conditions. In fact, acetone is being used as an acetylene stabilizer in high pressure tanks because acetylene by itself may explode at pressures higher that 15 psi.
http://navyaviation.tpub.com/14018/css/14018_642.htm
http://navyaviation.tpub.com/14018/css/14018_642.htm
Remember safety first! Check MSDS and consult with professionals before performing risky experiments.
-
popabiancamaria
- Sr. Member

- Posts: 29
- Joined: Thu Nov 24, 2011 2:21 pm
- Location: ROMANIA,Bucharest
I insist that acetone and acetylene will not react under ordinary conditions. Apparently, the authors of this problem forgot to indicate the conditions. I olnly guess that they meant the reaction in the presence of a base, such as in Favorskii–Babayan synthesis ketones and acetylenic compounds react in presence of alkali http://en.wikipedia.org/wiki/Acetylide or some other similar reaction with formation of acetylide first. The reaction of acetone and acetylide will give a tertiary alcohol

On the second stage this alcohol will dehydrate with sulfuric acid producing enyne http://en.wikipedia.org/wiki/Enyne that on the third stage will be hydrogenated into corresponding alkane isopentane
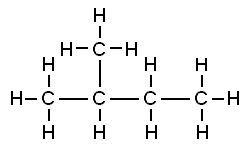

On the second stage this alcohol will dehydrate with sulfuric acid producing enyne http://en.wikipedia.org/wiki/Enyne that on the third stage will be hydrogenated into corresponding alkane isopentane
Remember safety first! Check MSDS and consult with professionals before performing risky experiments.
