Page 1 of 1
Trying to figure out reaction mechanism for reduction of nitro group.
Posted: Sat Nov 20, 2021 6:41 pm
by ScarletDemiurge
Hey, I'm trying to draw the mechanism for the catalytic hydrogenation of a NO2 group with Pd/C as a catalyst. I've looked at several articles (mostly "Insights into the mechanism of nitrobenzene reduction to aniline over Pd catalyst and the significance of the adsorption of phenyl group on kinetics" and "Studying the Mechanisms of Nitro Compounds Reduction (A-Review)") but they show slightly different paths to how NO2 turns into NH2. I drew the mechanism, but I'm not sure it is correct, could anyone give it a look and let me know if there's something wrong with it?
Note: I'm not sure if it's okay, but I'm assuming the hydrogen atoms that are on the surface of the Pd are able to be taken by other atoms as either protons (H+) or hydrides.
Re: Trying to figure out reaction mechanism for reduction of nitro group.
Posted: Sat Nov 20, 2021 9:07 pm
by Jatro
I think that there’s a problem with charge in the first step.
How can a neutral compound become negative after getting a proton?
If H is not a proton but a hydride, how can a negative oxygen attack a negative hydride?
Re: Trying to figure out reaction mechanism for reduction of nitro group.
Posted: Sat Nov 20, 2021 9:15 pm
by ScarletDemiurge
Do you think it would be better if in the first step the oxygen took the proton, but the other oxygen did not give its pi electrons to the positive nitrogen? That way the molecule in the second step would stay positive (due to the nitrogen's charge). The only thing is that if in the second step the oxygen with the double bond gives its pi electrons to the positive nitrogen, then in the third step the molecule would be negative and that isn't possible, is it? That a molecule goes from negative to positive charge all of a sudden, I mean.
Re: Trying to figure out reaction mechanism for reduction of nitro group.
Posted: Sat Nov 20, 2021 9:27 pm
by Jatro
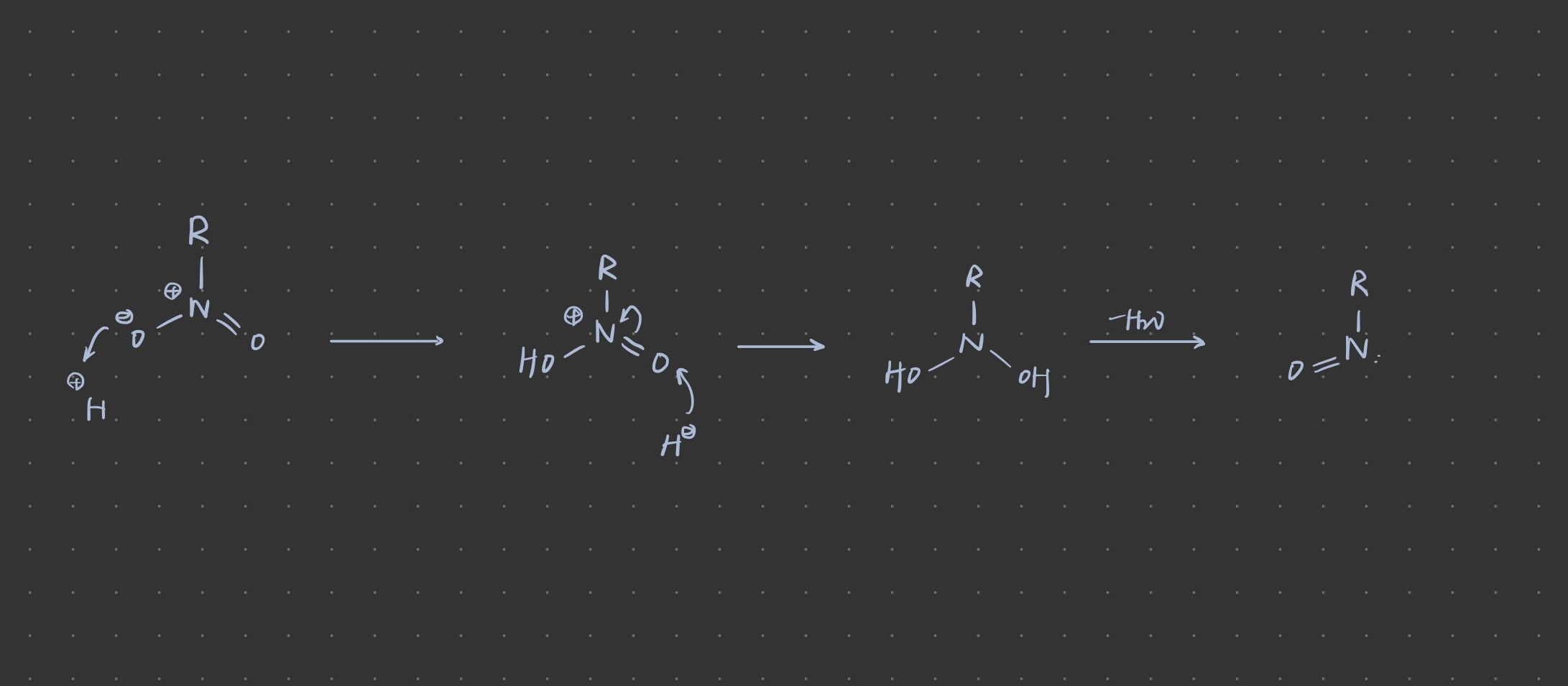
- IMG_1759.jpeg (98.53 KiB) Viewed 2592 times
After the oxygen gives its pi electron to nitrogen, the positive charge moves to oxygen and this molecule is still positive.
Re: Trying to figure out reaction mechanism for reduction of nitro group.
Posted: Sun Nov 21, 2021 10:59 am
by ScarletDemiurge
God, that's true, I don't know how that slipped my mind.
Thank you so much for your help!