Page 1 of 1
Total Synthesis
Posted: Tue Oct 19, 2021 9:11 am
by Alexi999
Does anyone have any ideas for a stepwise total synthesis of the following molecule C
3H
4NaO
7P?
Is it true that the dual carboxylate groups attached to the phosphate will retard hydrolysis in H
2O?
How can I do this?
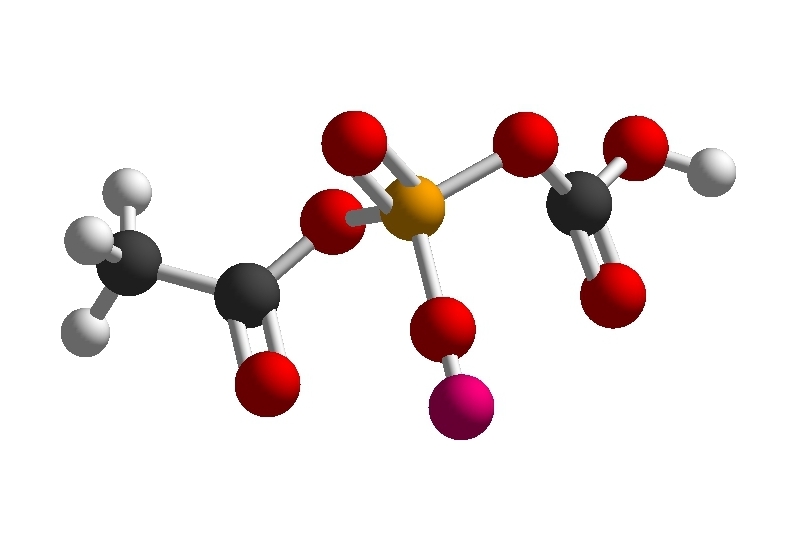
- Screenshot_20211019-104946_Molecular Constructor.jpg (65.99 KiB) Viewed 3040 times
Re: Total Synthesis
Posted: Tue Oct 19, 2021 10:06 am
by Alexi999
Could it be possible to design a simple electro-chemical cell that activates the carbonyl's to kick off (OH-)'s as leaving groups and at the same time pull up the P=O double bond to create a higher electrophilicity on P that holds the other single bonded oxygen closer to P allowing for a more positive affinity on phoshpate H's to grab the recently kicked off (OH-)'s
Any insight?
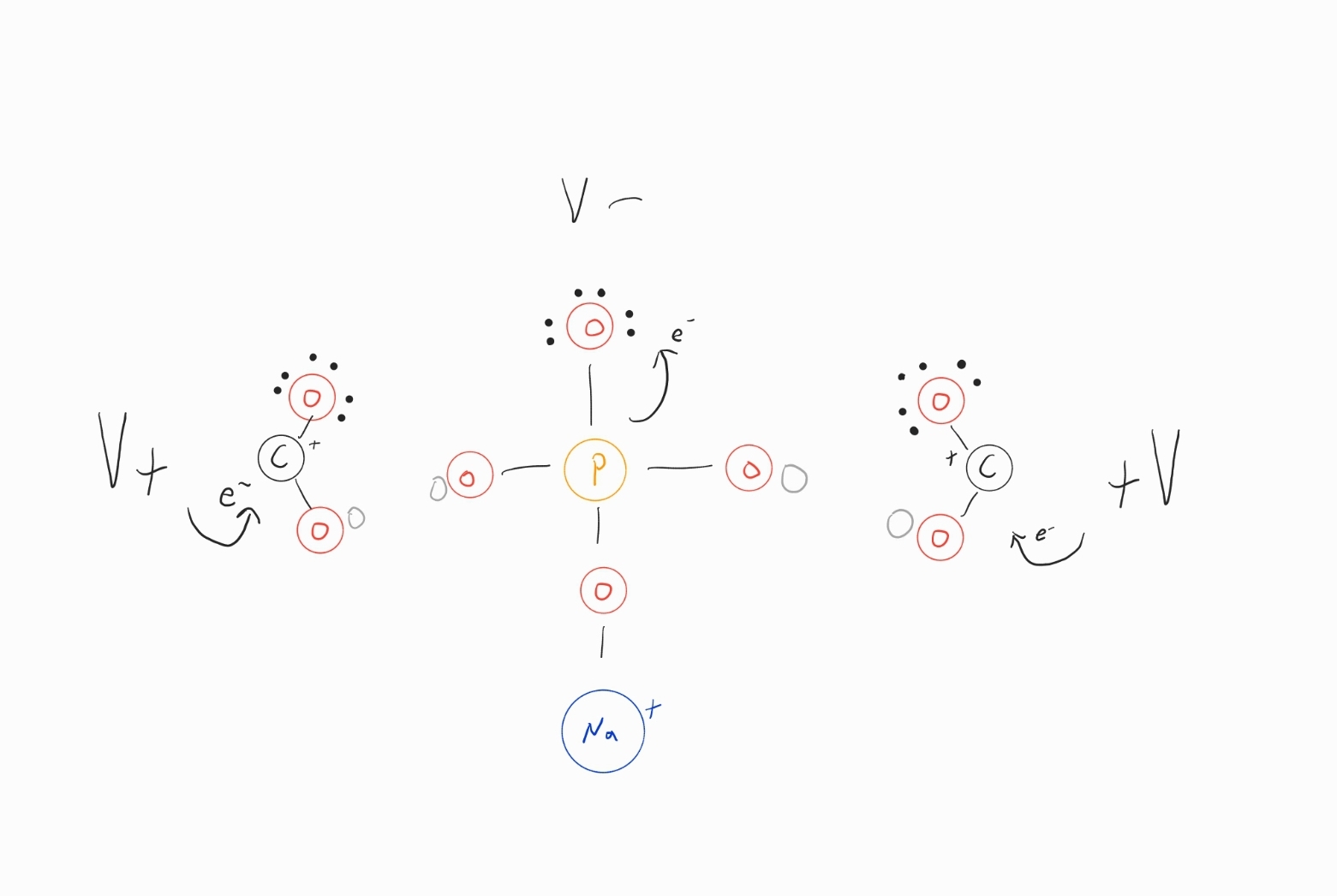
- Screenshot_20211022-095707_S Note.jpg (91.72 KiB) Viewed 2997 times
Re: Total Synthesis
Posted: Tue Oct 19, 2021 12:59 pm
by ChenBeier
Again a strange molecule. You have a combined anhydride of acetic acid , carbonic acid and phosphoric acid.
This will not exist. Carbonic acid will expell CO2. Then we have the same molecule what you asked before.
(CH3CO - OP(=O) - O(C=O)-OH)O- Na+
(CH3CO - OP(=O) - OH O- Na+ + CO2
Re: Total Synthesis
Posted: Fri Oct 22, 2021 7:21 am
by Alexi999
There is no adjunct to stabilize this molecule to prevent hydrolysis and CO2 decomposition in aqueous solution?
What about the isoelectric -HNO3?
Re: Total Synthesis
Posted: Fri Oct 22, 2021 7:54 am
by Alexi999
I know that in the biological system of humans there is an Zn based enzyme CA (carbonic anhydrase) that favors the formation of carbonic acid from H2O and CO2
Maybe a Zn adjunct complex would retard this decomposition?
Re: Total Synthesis
Posted: Fri Oct 22, 2021 8:05 am
by Alexi999
Basically, how can this dual oxoacid chained to a mono-phosphate salt be accomplished?
R1 O=C O-P-O C=O R2
Should be easy right?
Re: Total Synthesis
Posted: Fri Oct 22, 2021 8:36 am
by ChenBeier
These compounds , if existent are only stable without of presence of water.
Other acids like nitric will destable more.
What is the purpose to synthesise this compounds?