Page 1 of 1
Esterification Reaction
Posted: Sun Oct 17, 2021 1:29 pm
by Alexi999
I am interested to see if anyone knows the easiest way to mono-esterify a mono-phosphate salt group to a carboxylic acid.
How to facilitate the resultant -> product ?
CH
3COOH + NaH
2PO
4 ‐> C
2H
4NaO
5P
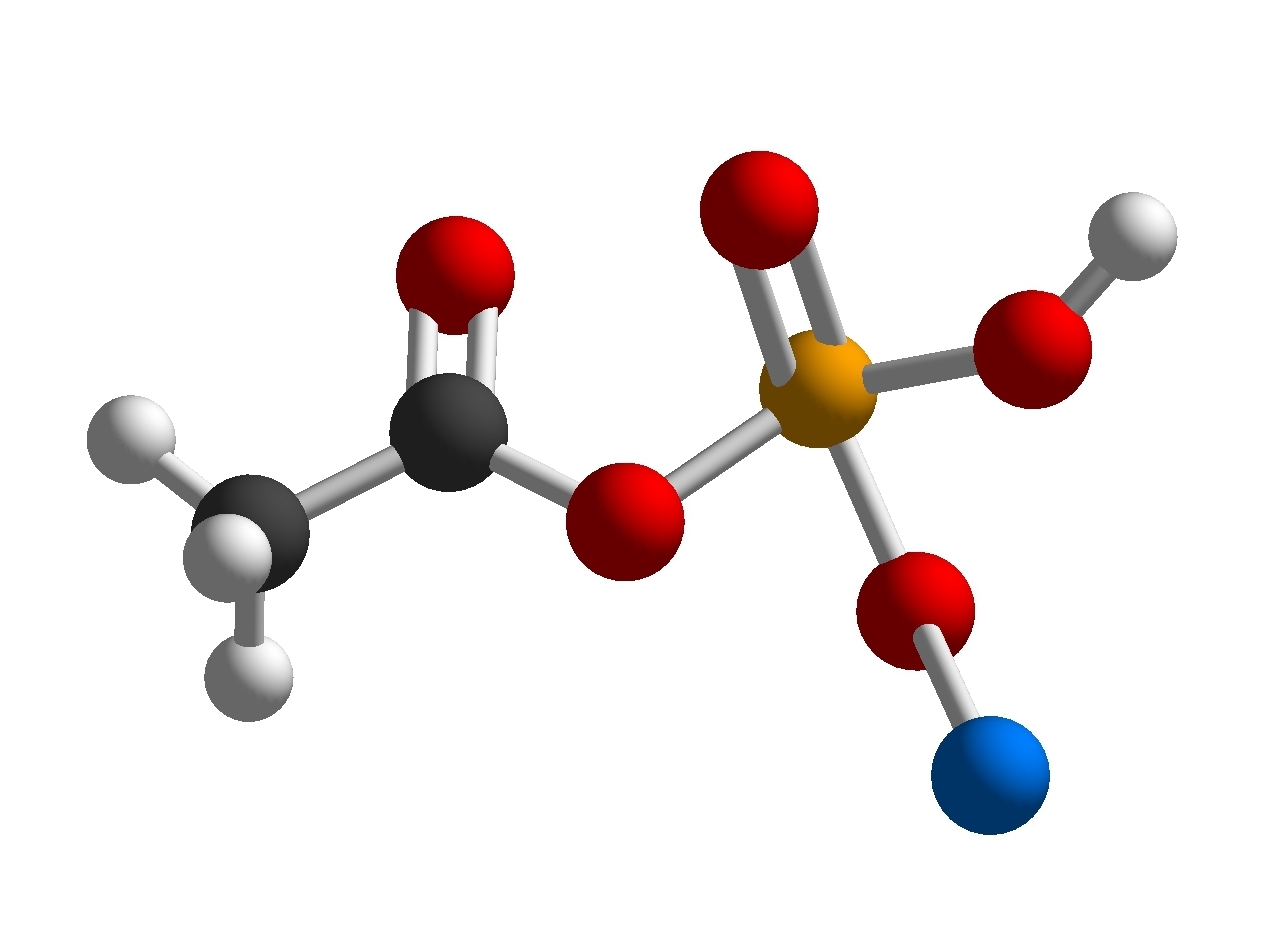
- Screenshot_20211017-151346_Molecular Constructor.jpg (118.44 KiB) Viewed 5226 times
Re: Esterification Reaction
Posted: Sun Oct 17, 2021 1:39 pm
by ChenBeier
First it's not a ester. Ester made from acid and alcohol.
What you talking about is a mixed acid anhydride.
To get this take acetylchloride CH3COCl and disodium hydrogen phosphate Na2HPO4.
CH3COCl + Na2HPO4 => CH3CONaHPO4 + NaCl
Re: Esterification Reaction
Posted: Sun Oct 17, 2021 3:02 pm
by Alexi999
Thank you for the reply!
I thought that an acid anhydride is a compound that has two acyl C(O)R groups bonded to the same oxygen atom.
Thanks for the clarification. I'm still learning.
Also, what prevents di-linkage at both sodium positions of the phosphate by acetylchloride?
Re: Esterification Reaction
Posted: Sun Oct 17, 2021 9:52 pm
by ChenBeier
The stoechometric mixture of the reactants guid to it. Probably take more of the phosphate.
Anhydride means remove water. The anhydride can made by 2 CH3COOH => (CH3CO)2O + H2O but also with different acids.
CH3COOH + C2H5COOH => CH3CO(C2H5CO)O + H2O
H3PO4 + CH3COOH => CH3COH2PO4 + H2O
Re: Esterification Reaction
Posted: Mon Oct 18, 2021 8:38 am
by Alexi999
Ok so treat the carboxylic acid with thionyl chloride ( SOCl2 ) to form the intermediate acid chloride in aqueous solution
Neutralize with NaOH
Then simply stoichiometrically add the disodium phosphate (Na2HPO4)
Then collect precipitate final product .
(What ratio of H2O to the CH3COOH do you suggest for optimal reactivity?)
Re: Esterification Reaction
Posted: Mon Oct 18, 2021 10:28 am
by ChenBeier
You can not use aqueous solution. SOCl2 either CH3COCl will decompose in presence of water.
Why to produce acetylchloride from acetic acid and thionylchloride, from cost side buy directly acetylchloride and add it to the dry phosphate.
Re: Esterification Reaction
Posted: Mon Oct 18, 2021 10:43 am
by Alexi999
So what if I want to use a different carboxylic acid, so what would be the system to do this
if I can't buy a chlorinated acid form?
Re: Esterification Reaction
Posted: Mon Oct 18, 2021 10:57 am
by Alexi999
So for example could I use sulfuric acid in aqueous solution to functionalize the carbonyl to
(CH3COOH + NaH2PO4) cat H2SO4 -> CH3CONaHPO4 +H2O
Re: Esterification Reaction
Posted: Mon Oct 18, 2021 11:03 am
by Alexi999
I'm just confused on how to go about this.
Re: Esterification Reaction
Posted: Mon Oct 18, 2021 12:03 pm
by ChenBeier
No, the product is water sensitive. The Anhydride will decompose if in contact with water. Sulfuric acid will any way convert the phosphate to phosphoric acid.
You can only make it in absense of water.
Of course if you cannot buy the chlorinated Acyl ,then you have to do the chlorination first.
Re: Esterification Reaction
Posted: Mon Oct 18, 2021 3:03 pm
by Alexi999
Yes, can you tell me the optimal route for this chlorination, for a full synthesis.
Re: Esterification Reaction
Posted: Mon Oct 18, 2021 9:42 pm
by ChenBeier
You already chosed it. Thionylchlorid SOCl2, but also phosphorous trichloride PCl3 or phosphorous oxychloride POCl3 are possible.
Re: Esterification Reaction
Posted: Tue Oct 19, 2021 5:31 am
by Alexi999
Thanks for you help.

Re: Esterification Reaction
Posted: Mon Nov 01, 2021 4:53 pm
by jebbasha
You have received tons of valuable advice here. Now you just really want to achieve the target. Theoretically, everyone wants to become slim, but no one realizes it is really hard work. I am from
https://worldcams.tv/spain/lanzarote/airport. And only when I stopped to feel sorry for myself, the things started to move. It the first step to take. Stop feeling sorry for yourself.