Page 1 of 1
Redox reactions
Posted: Sat Jul 24, 2021 5:48 am
by Dhamnekar Winod
How to answer all these following questions?
I am working on these questions. If you know the answers to these questions, you may post them. Why can't i paste the screenshot copied on clipboard here?
- electrontransfer reaction.png (51.96 KiB) Viewed 5103 times
Re: Redox reactions
Posted: Sat Jul 24, 2021 6:13 am
by ChenBeier
If you wish to attach one or more files enter the details below. You may also attach files by dragging and dropping them in the message box.
Maximum filesize per attachment: 1 MiB.
You have to have jpg or PDF.
For redox reaction use the redox pair law
a) S2O3 2- / S4O6 2- and I2/I-
2 S2O3 2- => S4O6 2-
Balance charge
2 S2O3 2- => S4O6 2- + 2e-
I2 => 2 I-
Balance charge
I2 + 2e- => 2 I-
Addition
2 S2O3 2- + I2=> S4O6 2- + 2 I-
Try the other by yourself.
Re: Redox reactions
Posted: Sun Jul 25, 2021 2:02 am
by Dhamnekar Winod
Answers to a), b) , C).
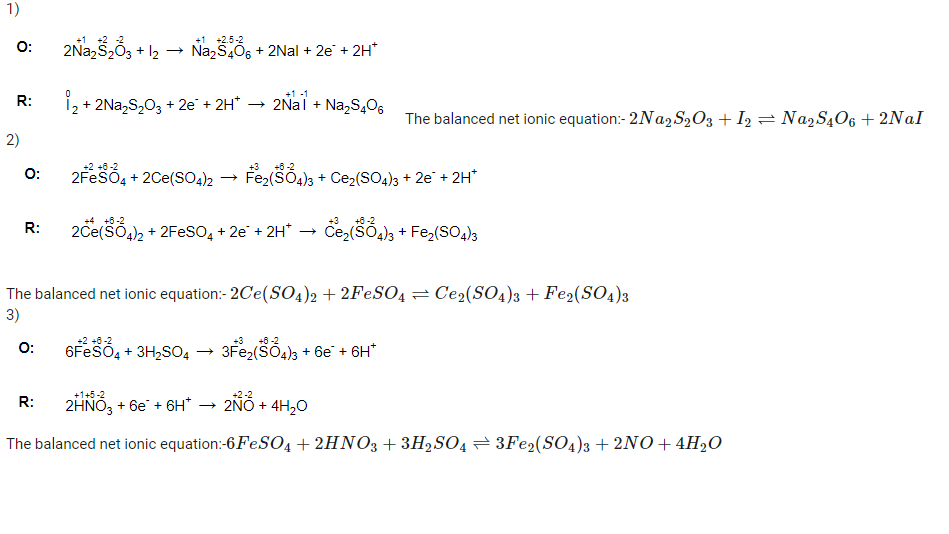
- RR123.png (29.1 KiB) Viewed 5077 times
Re: Redox reactions
Posted: Sun Jul 25, 2021 6:29 am
by ChenBeier
The final results are ok, I am wondering where the 2 H+ + 2e- comming from. Especially there is no acid in 1. and 2.
Fe 2+ => Fe 3+ + e- Oxidation
Ce 4+ +e - => Ce 3+ Reduction
Fe 2+ + Ce 4+ => Fe 3+ + Ce 3+ Addition
Sulfate is spectator ion
For completion multiply by 2 and add SO4 2-
2 FeSO4 + 2 Ce(SO4)2 => Fe2(SO4)3 + Ce2(SO4)3
Fe 2+ => Fe 3+ + e- Oxidation
HNO3 + 3 H+ + 3e- => 2 H2O + NO Reduction
Multiplikation of Oxidation by 3
3 Fe 2+ + 3 Fe 3+ + 3 e-
3 Fe 2+ + HNO3 + 3 H+ => 3 Fe 3+ + 2H2O + NO Addition
Also hier sulfate is spectator but can be added accordingly
6 FeSO4 + 2HNO3 + 3 H2SO4 => 3 Fe2(SO4)3 + 4 H2O + 2 NO
Re: Redox reactions
Posted: Wed Jul 28, 2021 12:32 am
by Dhamnekar Winod
Answers to d), e), f), g).

- RRdefg.png (36.97 KiB) Viewed 5025 times
Re: Redox reactions
Posted: Wed Jul 28, 2021 2:39 am
by ChenBeier
I dont understand how you develop the part equations. In e) for example you use OH- even the reaction takes place with HCl. With mathematics a lot can do even the final result is correct but generally its wrong.
To start like this
d) S 2- => S + 2 e- Oxidation
HNO3 + 3 H+ +3 e- => NO + 2 H2O Reduction
e) S 2- => S+ 2 e- Oxidation
Cr2O7 2- + 6 HCl + 8 H+ + 6 e- => 2 Cr 3+ + 7 H2O + 6 Cl- Reduction
f) Cl2 + 12 OH- => 2 ClO3- + 6 H2O + 10 e- Oxidation
Cl2 + 2 e- => 2 Cl- Reduction
g) Fe 2+ => Fe 3+ + e- Oxidation
O2 2- + 2H2O + 2 e- => 4 OH- Reduction
Re: Redox reactions
Posted: Wed Jul 28, 2021 8:29 am
by Dhamnekar Winod
In answer to e) , I used basic medium even though HCl is reactant. You said it is wrong. After using acidic medium, the answer is as follows:

- RRehij.png (20.52 KiB) Viewed 5011 times
The balanced net ionic equations for h), i), j) are as follows:
h)
\(Bi_2S_3 + 8HNO_3 \rightleftarrows 2Bi(NO_3)_3 + 2NO + 3S + 4H_2O\)
i)
\(H_3AsO_4 + 4Zn + 8HNO_3 \rightleftarrows AsH_3 + 4Zn(NO_3)_2 + 4H_2O\)
j)
\(2CrI_3 + 64NaOH + 27Cl_2 \rightleftarrows 2Na_2CrO_4 + 6NaIO_4 + 54NaCl + 32H_2O\)