Page 1 of 1
Molecular structures
Posted: Sat Jun 05, 2021 1:51 am
by Dhamnekar Winod
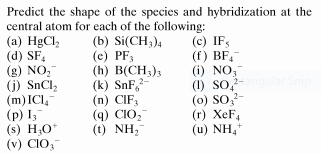
- Molecularstructure.PNG (18.91 KiB) Viewed 1825 times
I am trying to predict the shape of species and hybridization at the central atom for all chemical compounds in this question. Let me work on it.
Re: Molecular structures
Posted: Mon Jun 07, 2021 3:58 am
by Dhamnekar Winod
(a)Linear sp (b) Tetrahedral sp³ (c)Square Pyramid sp³d² (d) See-saw sp³d² (e) Pyramidal sp³ (f) Tetrahedral sp³
(g) Bent sp² (h) Trigonal Planar sp² (i) Trigonal Planar sp² (j) Bent sp² (k) Octahedral sp³d² (l) Tetrahedral sp³
(m) Square Planar sp³d² (n) T-shape sp³d (o)Pyramidal sp³ (p) Linear sp³d (q) Bent sp³ (r) Square Planar sp³d²
(s) Pyramidal sp³ (t) Bent sp³ (u) Tetrahedral sp³ (v) Pyramidal sp³
In my opinion, above-mentioned all answers to this questions are correct.