Page 2 of 2
Re: Titration and pH computation
Posted: Fri Apr 30, 2021 12:12 pm
by ChenBeier
Please show the total calculation. I dont have the time to recalculate everything.
Re: Titration and pH computation
Posted: Sat May 01, 2021 7:02 am
by Dhamnekar Winod
Computation of pH of 0.050 L C
6H
15N and HCl mixture,
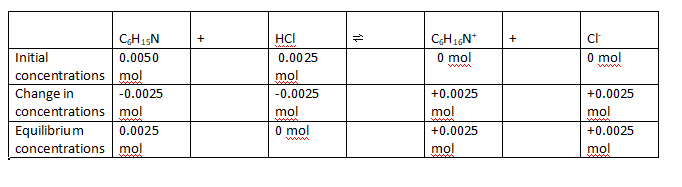
- ICEchart.png (7.4 KiB) Viewed 1611 times
Using Handerson HasselBalch equation,
pH = pKa + log (cB/cA) , where c= concentration, B= base, A=acid
we get pH= 10.72 + log(1)= 10.72
Re: Titration and pH computation
Posted: Sat May 01, 2021 7:26 am
by ChenBeier
Looks good now. Before you got 6.1