Page 1 of 1
Stoichiometry % Yields and Reactants
Posted: Sun Mar 28, 2021 8:52 am
by sheilamrohrbach
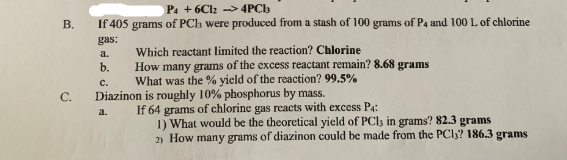
- sos.png (124.51 KiB) Viewed 1623 times
Can someone please help me? I've been trying to solve this since last night but my numbers are just so far.

Re: Stoichiometry % Yields and Reactants
Posted: Sun Mar 28, 2021 9:55 am
by ChenBeier
All results are correct. What is the problem.?
Re: Stoichiometry % Yields and Reactants
Posted: Sun Mar 28, 2021 10:30 am
by sheilamrohrbach
I need the solution. I've been trying since last night but my numbers are off

Re: Stoichiometry % Yields and Reactants
Posted: Sun Mar 28, 2021 11:11 am
by ChenBeier
For a) you calculate the mol of 405g PCl3 and according the equation you calculate how much Chlorine and phosphorous you need. Then you calculate the 100g P4 and 100 l Cl2 and compare. Dont forget the stiochometric factors.
n =m/M, n = mass/ molar mass.
b) is the difference of the theoretic and practical value.
c) similar, practical value of producct divided by theoretisc value of product
d) similar calculation with new values
e) dito
