Page 1 of 1
Ortho-fluoro aniline and pH reactions
Posted: Sat Mar 20, 2021 3:22 am
by Dhamnekar Winod
Consider ortho-fluoro aniline (o-FC6H4NH2).
a.Give an electron-dot formula for this compound and indicate with an arrow which atoms can accept a proton. Circle the atom that actually does accept a proton from water.
b.Determine whether o-FC6H4NH2 is a stronger or weaker base than aniline itself (C6H5NH2) and explain why this is the case.
c.Calculate the pH of a 0.1 M solution of o-FC6H4NH2.
d.Draw the structural formula of the conjugate acid of o-FC6H4NH2.
e.Calculate the pH of a 0.010 M solution of the conjugate acid of o-FC6H4NH2.
What are answers to all these questions? How to answer all these questions? Let me answer all these questions one by one.
Re: Ortho-fluoro aniline and pH reactions
Posted: Sat Mar 20, 2021 11:22 pm
by Dhamnekar Winod
Answer to a.
Chemical structure of ortho-fluoro aniline is as follows:
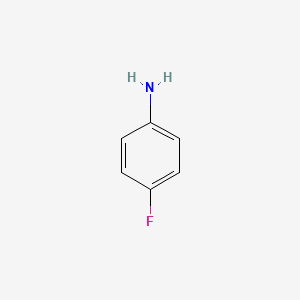
Nitrogen atom can accept proton from water.
Answer to b.
o-FC
6H
4NH
2 is stronger base than C
6H
5NH
2 because F can act as base (electron pairs donor)
Re: Ortho-fluoro aniline and pH reactions
Posted: Sun Mar 21, 2021 1:07 am
by ChenBeier
Both are wrong . What you draw is para fluor aniline not the ortho one.
And anilin pka 4,6 is stronger base as the fluoraniline pka 3,2. Reason strong - I effect of flourine, M - effect is miner.
Re: Ortho-fluoro aniline and pH reactions
Posted: Sun Mar 21, 2021 3:41 am
by Dhamnekar Winod
Are Yoy talking about mesomeric effect and inductive effect of Fluorine?
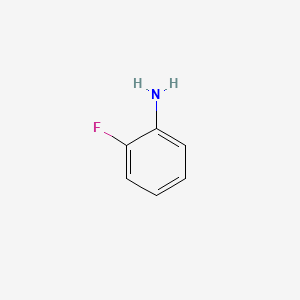
Is this correct chemical structure drawing for ortho-fluoro aniline?
Re: Ortho-fluoro aniline and pH reactions
Posted: Sun Mar 21, 2021 4:14 am
by ChenBeier
Now it is correct. Yes i talk about inductive and mesomeric effect.
Re: Ortho-fluoro aniline and pH reactions
Posted: Sun Mar 21, 2021 8:24 am
by Dhamnekar Winod
Fluorine atom will accept proton from water in ortho-fluoro aniline.
You said pKa of the o-fluoro aniline is 3.2, so its pH is 3.2 as well.
Now, how to answer part d and e?
Re: Ortho-fluoro aniline and pH reactions
Posted: Sun Mar 21, 2021 9:32 am
by ChenBeier
Wrong. The only atom which accept proton will be nitrogen to form the ammonium.
The calculation is similar the first exercise with the trimethylamin.
Re: Ortho-fluoro aniline and pH reactions
Posted: Sun Mar 21, 2021 11:24 pm
by Dhamnekar Winod
Let me answer part e.
Conjugate acid of o-FC6H4NH2 is o-FC6H4NH3
Reaction equation is FC6H4NH2 + H2O → FC6H4NH3⁺ + OH⁻
So, 1.6E-11=x*x/0.01M → x= 4.0e-7, Hence pOH=-log(4.0e-7)= 6.4. so, pH=14.0-6.4=7.6 Is this computaion correct?
Re: Ortho-fluoro aniline and pH reactions
Posted: Mon Mar 22, 2021 4:07 am
by ChenBeier
Where does the 0,00625 coming from. pH should be alkaline because its a amin
Re: Ortho-fluoro aniline and pH reactions
Posted: Mon Mar 22, 2021 4:55 am
by Dhamnekar Winod
I edited the answer. Please check it out.
Re: Ortho-fluoro aniline and pH reactions
Posted: Mon Mar 22, 2021 6:08 am
by ChenBeier
I dont know what you doing, where this numbers come from. pKa is 3.2 already
Re: Ortho-fluoro aniline and pH reactions
Posted: Mon Mar 22, 2021 6:19 am
by Dhamnekar Winod
If pKa is 3.204, then ka= 0.000625, this means 0.000625* 0.01= x * x , → x= 0.0025 Now is this hydroxide ion concentration? If it is so, then poH=-log(0.0025)=2.602, this means pH= 14.000 -2.602=11.398 Is this computation correct?
Re: Ortho-fluoro aniline and pH reactions
Posted: Mon Mar 22, 2021 6:35 am
by ChenBeier
Looks better now
Re: Ortho-fluoro aniline and pH reactions
Posted: Fri Mar 26, 2021 5:21 am
by Dhamnekar Winod
How to use pH solver available on webqc.org to answer this question?
Re: Ortho-fluoro aniline and pH reactions
Posted: Fri Mar 26, 2021 6:52 am
by ChenBeier
The given pKs is 3.2 for FC6H4NH3+ , for the solver has to be used the corresponding pKb value of 10.8.
pH about 8.1
 Nitrogen atom can accept proton from water.
Nitrogen atom can accept proton from water. Is this correct chemical structure drawing for ortho-fluoro aniline?
Is this correct chemical structure drawing for ortho-fluoro aniline?