Page 1 of 1
Pentenol (right?) vs. Pentone (??) and why?
Posted: Sun May 11, 2008 8:02 pm
by MorganFreeman2
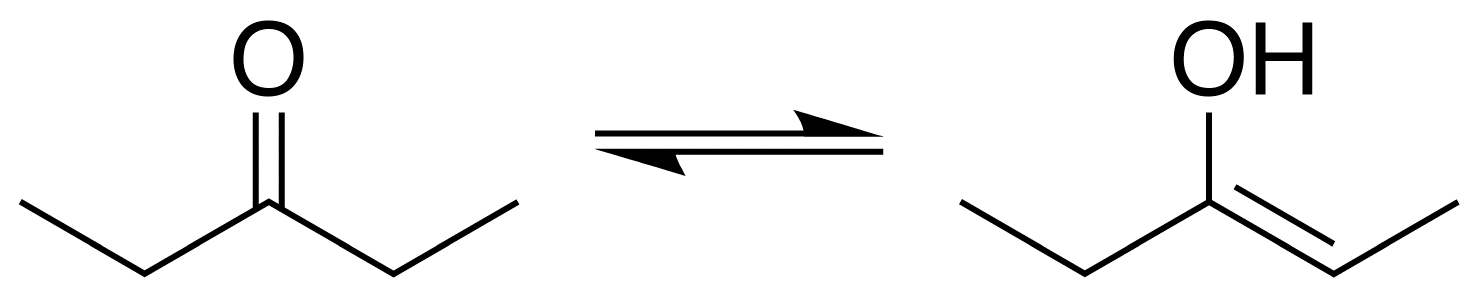
Why is the second compound more stable than the first?
Posted: Mon May 12, 2008 4:01 pm
by Zedekiah
Oxygen is more electronegative and therefore makes stronger double bonds than carbon.
Posted: Mon May 12, 2008 10:05 pm
by MorganFreeman2
Is it because the oxygen has less pulling on it's electrons when it's double bonded with carbon, than with an hydrogen and carbon?
Posted: Tue May 13, 2008 5:39 am
by Zedekiah
I think that is fair to say. It want electrons more than carbon does, so it will be more stable with the double bond than carbon will be.

